
These ones should be as deep as possible to achieve a high open-circuit voltage, ii) the mobility of charge carriers in the semiconductor. Namely, the overall requisites for an efficient p-SC are: i) the position in energy of the uppermost levels of its valence band. To cope with this situation, many researches devoted to the quest of new p-type semiconductors as substitutes of NiO, the commonly used material, have been initiated. In contrast, p-DSSCs still exhibit very low conversion yields and have to be regarded as the bottleneck that limits the development of tandem DSSCs. Nowadays, n-DSSCs with high performances have been achieved. Indeed, such a device represents a promising way to harvest a maximum of the incident light, and then optimize the conversion efficiency of solar energy into electric power, ,, , ]. The redox mediator solubilized in the electrolyte ensures the charge flow within the solar cell. Under illumination, adsorbed sensitizers are excited and inject charges (electrons and holes) into the metal oxide semiconductor (SC). Both electrodes are dye coated and separated by an electrolyte. TiO 2 and ZnO) and a photo-cathode built upon a p-type semiconductor ( e.g. Photovoltaic activity was confirmed with DPP-NDI dye as sensitizer and tris(4,4′-ditert-butyl-2,2′-bipyridine)cobalt(III/II) as redox mediator.Ī tandem dye sensitized solar cell (DSSC) consists of a photo-anode built upon an n-type semiconductor ( e.g. These nanoparticles were then deposited as a film on a FTO glass by screen printing and used after sintering as photocathodes for p -DSSCs.

Both techniques concluded to the p -type conductivity of the Cu 2 hetero-structures.
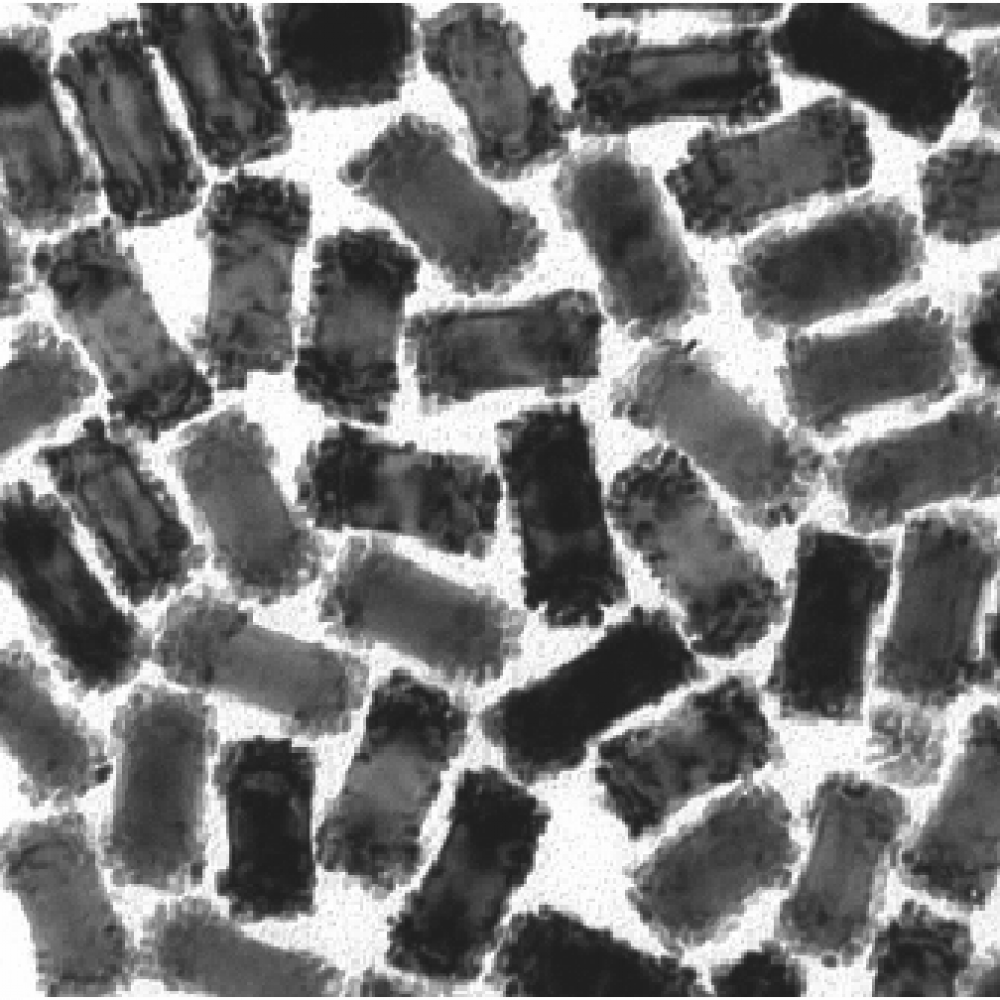
The as-obtained Cu 2 nanoparticles were characterized by X-ray photoelectron spectroscopy and electrochemical impedance spectroscopy. This shell may be viewed as a passivating layer that overcomes the natural chemical instability of Cu 2O towards electrolytes for instance. From HRTEM micrographs, it was evidenced that Cu 2O particles were uniformly covered by a 5 nm thick CuO layer to form a core-shell structure. Cu 2 core-shell nanoparticles were synthesized via a facile precipitation-thermal oxidation method consisting in the preparation of Cu 2O nanoparticles in solution followed by a post-treatment at 300 ☌, 350 ☌ or 400 ☌ in air.


 0 kommentar(er)
0 kommentar(er)
